Parallel dilution is the dilution of a solution with equal quantity of the same solvent with which the solution is made. E.g., 1mL of 100µg/ml strength aqueous solution can be diluted to 2mL of. Serial and Parallel Dilution A serial dilution is a stepwise preparation in which each dilution serves as the source for the next dilution. Serial Dilutions. A dilution series is a succession of step dilutions, each with the same dilution factor, where the diluted material of the previous step is used to make the subsequent dilution. This is how standard curves for ELISA can be made. To make a dilution series, use the following formulas: Move Volume = Final Volume / (DF -1). Serial dilution Definition. Serial dilution, as the name suggests, is a series of sequential dilutions that are performed to convert a dense solution into a more usable concentration. In simple words, serial dilution is the process of stepwise dilution of a solution with an associated dilution factor.
- Serial Vs Parallel Dilution Method Pdf
- Parallel Dilution Formula
- Serial Vs Parallel Dilution Method Calculator
- Serial Vs Parallel Dilution Method To Pass. With serial dilutions, a concentrated sample is diluted down in a stepwise manner to make lower concentrations. Hindi Fonts Converter is a product developed by Window India.This site is not directly affiliated with Window India.All trademarks, registered trademarks, product names and company names.
- The serial dilution method is standard practice in the preparation of doseresponse series for IC50 determination. However, it is well recognised that inadequacies in the liquid handling or mixing technique will affect the dilution ratio and hence the compound concentration and any errors will be compounded during each successive serial dilution, mix and transfer.
Serial Vs Parallel Dilution Method Pdf
Introduction
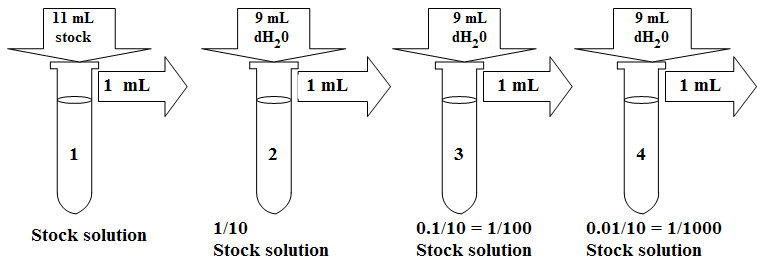
A Serial dilution is a series of dilutions, with the dilution factor staying the same for each step. The concentration factor is the initial volume divided by the final solution volume. The dilution factor is the inverse of the concentration factor. For example, if you take 1 part of a sample and add 9 parts of water (solvent), then you have made a 1:10 dilution; this has a concentration of 1/10th (0.1) of the original and a dilution factor of 10. These dilutions are often used to determine the approximate concentration of an enzyme (or molecule) to be quantified in an assay. Serial dilutions allow for small aliquots to be diluted instead of wasting large quantities of materials, are cost-effective, and are easy to prepare.
Equation 1.
[concentration factor= frac{volume_{initial}}{volume_{final}}nonumber]
[dilution factor= frac{1}{concentration factor}nonumber]
Key considerations when making solutions:
- Make sure to always research the precautions to use when working with specific chemicals.
- Be sure you are using the right form of the chemical for the calculations. Some chemicals come as hydrates, meaning that those compounds contain chemically bound water. Others come as “anhydrous” which means that there is no bound water. Be sure to pay attention to which one you are using. For example, anhydrous CaCl2has a MW of 111.0 g, while the dehydrate form, CaCl2 ● 2 H2O has a MW of 147.0 grams (110.0 g + the weight of two waters, 18.0 grams each).
- Always use a graduate cylinder to measure out the amount of water for a solution, use the smallest size of graduated cylinder that will accommodate the entire solution. For example, if you need to make 50 mL of a solution, it is preferable to use a 50 mL graduate cylinder, but a 100 mL cylinder can be used if necessary.
- If using a magnetic stir bar, be sure that it is clean. Do not handle the magnetic stir bar with your bare hands. You may want to wash the stir bar with dishwashing detergent, followed by a complete rinse in deionized water to ensure that the stir bar is clean.
- For a 500 mL solution, start by dissolving the solids in about 400 mL deionized water (usually about 75% of the final volume) in a beaker that has a magnetic stir bar. Then transfer the solution to a 500 mL graduated cylinder and bring the volume to 500 mL
- The term “bring to volume” (btv) or “quantity sufficient” (qs) means adding water to a solution you are preparing until it reaches the desired total volume
- If you need to pH the solution, do so BEFORE you bring up the volume to the final volume. If the pH of the solution is lower than the desired pH, then a strong base (often NaOH) is added to raise the pH. If the pH is above the desired pH, then a strong acid (often HCl) is added to lower the pH. If your pH is very far from the desired pH, use higher molarity acids or base. Conversely, if you are close to the desired pH, use low molarity acids or bases (like 0.5M HCl). A demonstration will be shown in class for how to use and calibrate the pH meter.
- Label the bottle with the solution with the following information:
- Your initials
- The name of the solution (include concentrations)
- The date of preparation
- Storage temperature (if you know)
- Label hazards (if there are any)
Lab Math: Making Percent Solutions
Equation 2.
Formula for weight percent (w/v):

[ dfrac{text{Mass of solute (g)}}{text{Volume of solution (mL)}} times 100 nonumber ]
Example
Parallel Dilution Formula
Make 500 mL of a 5% (w/v) sucrose solution, given dry sucrose.
Serial Vs Parallel Dilution Method Calculator
- Write a fraction for the concentration [5:%: ( frac{w}{v} ): =: dfrac{5: g: sucrose}{100: mL: solution} nonumber]
- Set up a proportion [dfrac{5: g: sucrose}{100: mL: solution} :=: dfrac{?: g: sucrose}{500: mL: solution} nonumber]
- Solve for g sucrose [dfrac{5: g: sucrose}{100: mL: solution} : times : 500 : mL : solution : = : 25 : g : sucrose nonumber]
- Add 25-g dry NaCl into a 500 ml graduated cylinder with enough DI water to dissolve the NaCl, then transfer to a graduated cylinder and fill up to 500 mL total solution.



